An innovative method developed by scientists in South Korea allows the creation of diamonds in just 15 minutes, overcoming the limitations of traditional methods and opening up new possibilities for the gem industry.
@Nature
Diamonds created in a lab in only 15 minutes? This is now a reality thanks to an innovative technique developed in South Korea. Scientists have pioneered a method that could revolutionize the traditional diamond market by making production faster, cheaper, and more sustainable.
For decades, lab-grown diamonds have been produced by replicating the Earth’s mantle, an enormous task requiring immense pressures and scorching temperatures to transform carbon into synthetic diamonds. This method, known as High Pressure High Temperature (HPHT) growth, is not only extremely energy and time-intensive (taking weeks) but also yields limited results. HPHT diamonds only grow to the size of a blueberry and struggle to produce larger gems.
The new technique developed by Dr. Rodney Ruoff and his team at the Institute for Basic Science in South Korea eliminates these limitations. Instead of replicating the Earth’s fiery furnace, they devised a surprisingly simple method that operates at atmospheric pressure. The key lies in a specially designed chamber and the use of gallium, a metal that catalyzes the formation of graphene from methane.
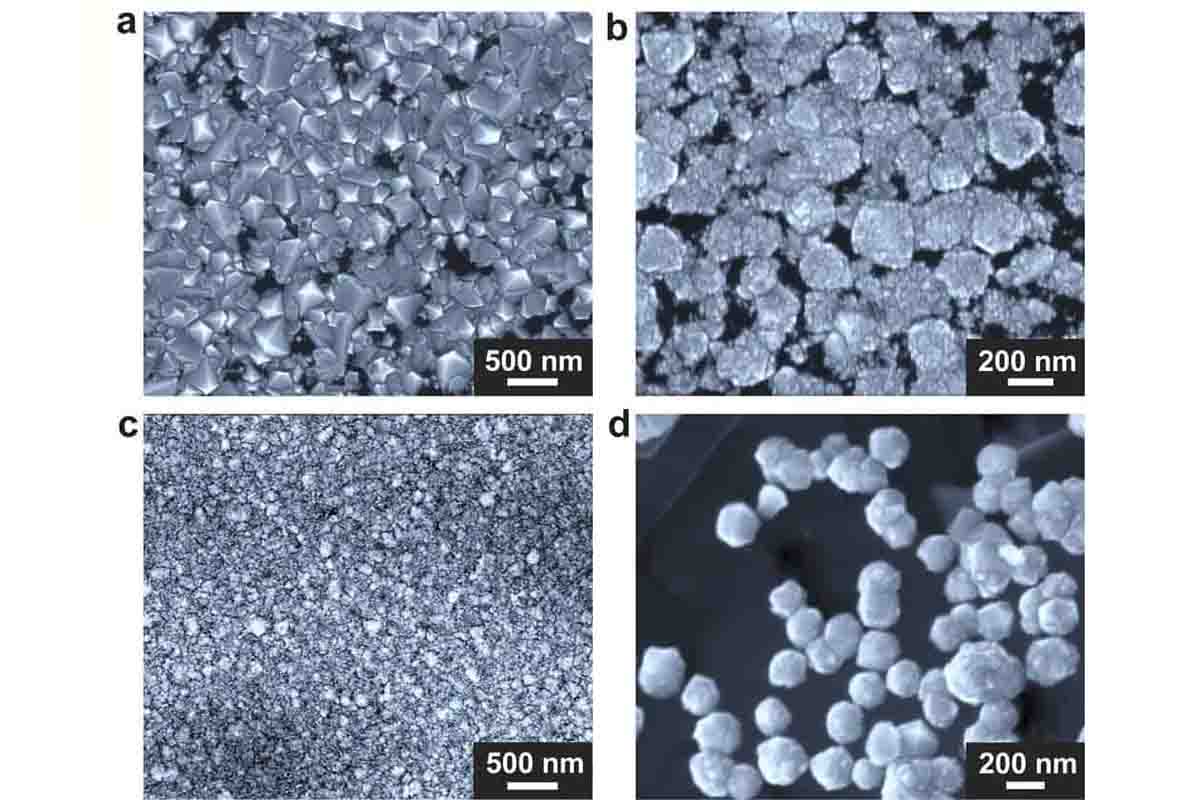
During experiments, researchers channeled carbon-rich methane gas, super-heated, through the special chamber. Here, the gas met a crucible containing a unique mix of gallium, nickel, iron, and a dash of silicon. In just fifteen minutes, diamond deposits materialized at the bottom of the crucible.
The resulting diamonds are almost pure, consisting mainly of carbon with a few silicon atoms as impurities. Although the exact mechanisms are still under study, researchers believe a temperature drop inside the chamber concentrates the carbon, inducing it to crystallize into diamonds. Silicon seems to play a crucial role in this process, possibly acting as a seed for diamond formation, as Dr. Ruoff explains:
“For over a decade, I’ve been thinking of new ways to grow diamonds, believing it could be done in unexpected ways compared to conventional thinking. In about a year or two, the world might have a clearer picture of the potential commercial impact.”
However, there is a drawback. Although the new method boasts incredible speed and simplicity, it produces microscopic diamonds, too small to adorn a finger or a necklace. But there is a silver lining. The low-pressure environment used in this technique makes scientists optimistic about scaling up production. If they can grow these diamonds to usable sizes, it could be a game-changer for the industry.
So, what does the future hold for these tiny diamonds? While they might not dazzle on your finger in the near future, their industrial potential is vast. In the future, new diamonds could be just fifteen minutes away.
Source: Nature
