In response to the increase in battery waste, Polish scientists propose an innovative approach to recycle lithium ions and turn them into catalysts for the production of hydrogen peroxide
Imagine opening a drawer filled with old used-up batteries, the kind with which one would not give a second thought before discarding. Well, what if I told you that in them lies a treasure? That is what a group of Polish researchers found out-turning hazardous waste into an unexpected resource.
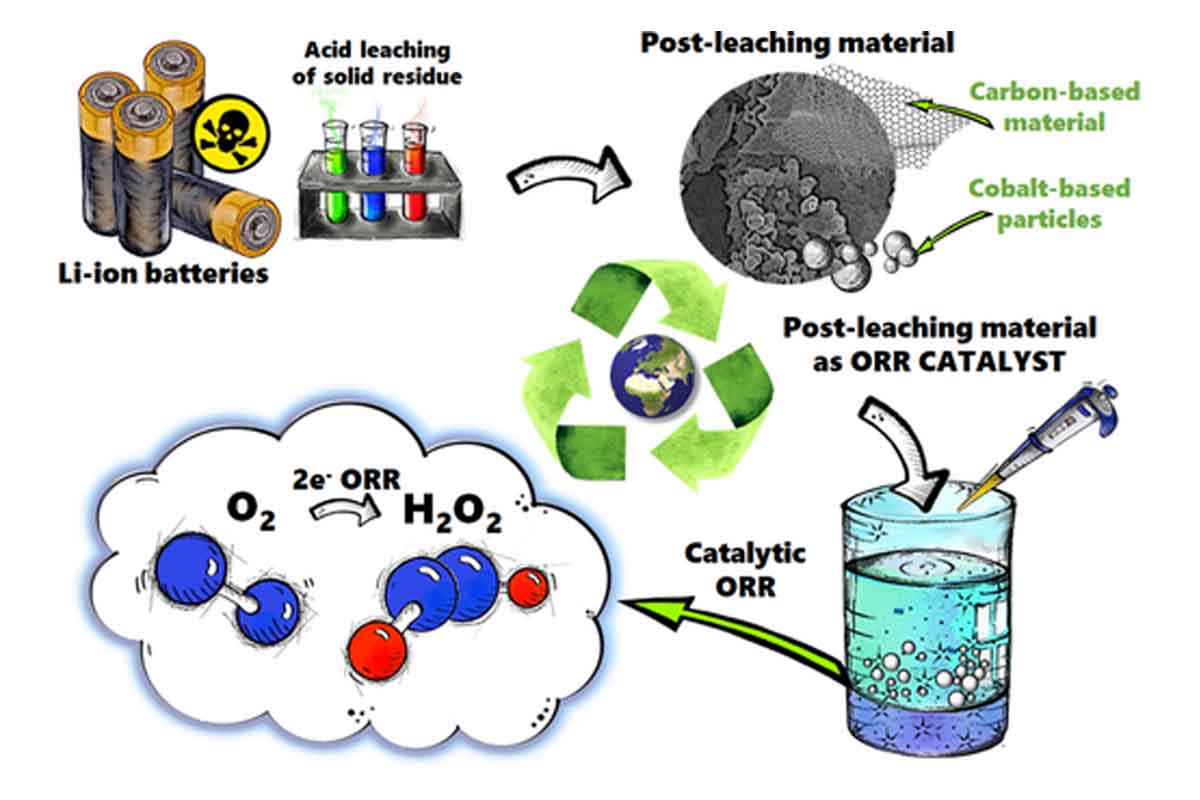
The research, published by ChemElectroChem, revealed that electrodes within lithium-ion batteries contain precious materials such as cobalt that can be recovered through the acid leaching process. The real stroke of genius is using leftover carbon, contaminated with metal traces, as a catalyst for the production of hydrogen peroxide, a product very much at the heart of many industries.
This re-presentation means that this supposed waste carbon powder has shown its usefulness for catalyzing certain chemical reactions. One of the most promising uses could be its application for the production of hydrogen peroxide. Now, hydrogen peroxide is a widely chemically known compound because of its popular application as a disinfectant, but it is also very important in many industries. Classic production of hydrogen peroxide requires very high temperatures, high pressure, and expensive catalysts. The approach is revolutionary because, with materials recovered from batteries, the Polish researchers managed to produce hydrogen peroxide in a more environmentally friendly way, with considerable cost savings.
How did they do this?
©ChemElectroChem
These wastes, which contained carbon and cobalt, served as electrochemical catalysts for the experts. These catalysts could allow the reduction of oxygen into hydrogen peroxide during electrochemical tests. There is, however, a catch: the catalytic properties of the material strongly depend on the chemical composition and structure of the recovered carbon, which in turn depends on the conditions of the leaching process. This means, in effect, that the methodology of extraction and processing needs further refinement in order to enhance the efficiency of the process.
Hydrogen peroxide is one of the basic chemical molecules and indispensable in many industries. Generally, its large-scale production requires high pressure and temperature, expensive catalysts, and different toxic electrolytes.
“Our aim was to work out a more ecological route for hydrogen peroxide production, especially an electrochemical one, with the use of catalysts made from post-industrial waste of lithium-ion batteries,” says Dr. Eng. Magdalena Warczak (PBS).
Why does this matter?
This technology recycles used batteries, saves tons of hazardous materials from burial at landfills, and opens completely new perspectives and ways for further use regarding the materials recovered. It is not just a question of reducing environmental impact, but rather of opening real opportunities in a range of industries well beyond simple recycling. Such valorized secondary raw materials could be used not only in hydrogen peroxide production but also in many important chemical reactions, opening perspectives for really new sectors like the space industry.
Source: ChemElectroChem
